Podcast- Antonio Abbate, MD & Ben Van Tassell, PharmD - Interleukin-1 (IL-1) Blockade in Acute Myocardial Infarction ...
Antonio Abbate, MD & Ben Van Tassell, PharmD work at Virginia Commonwealth University. In this podcast Dr. Abbate and Dr. Van Tassell speak on the Interleukin-1 (IL-1) Blockade in Acute Myocardial Infarction (VCU-ART3) (VCU-ART3) trial.
https://clinicaltrials.gov/ct2/show/NCT01950299?recrs=e&cond=Heart&phase=23&lupd_s=01%2F01%2F2019&lupd_e=01%2F03%2F2022&draw=3&rank=590
Abstract-
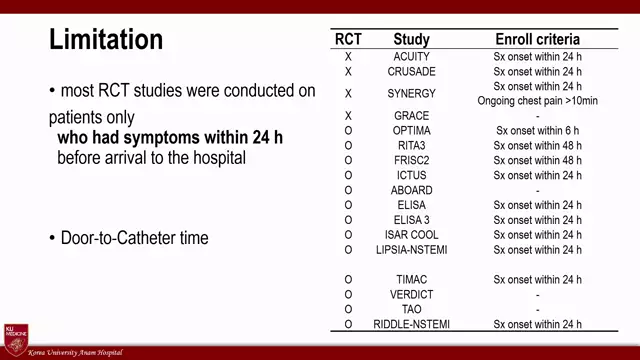
VCU-ART3 is a randomized double-blind clinical trial in patients with ST-segment elevation myocardial infarction (STEMI) that compares the impact of anakinra high dosage vs. anakinra standard dose vs. placebo on the acute rise and fall of plasma C reactive protein levels over the first 14 days.
Description in detail:
VCU-ART3 is a randomized double-blind clinical trial in patients with ST-segment elevation myocardial infarction (STEMI) that compares the impact of anakinra high dosage vs. anakinra standard dose vs. placebo on the acute rise and fall of plasma C reactive protein levels over the first 14 days.